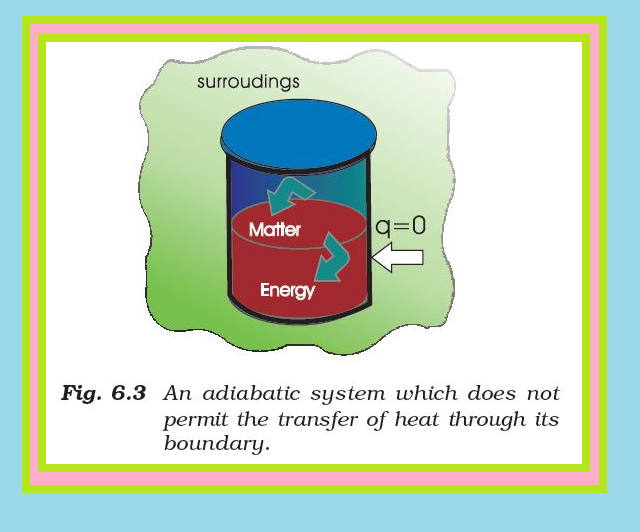
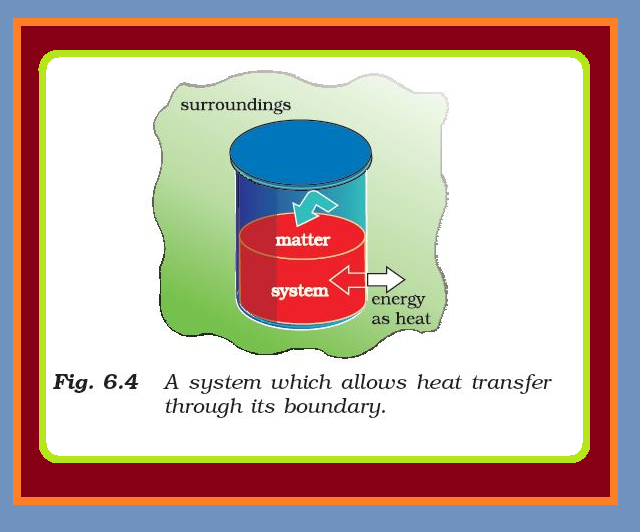
The State of the System :
`=>` The system must be described in order to make any useful calculations by specifying quantitatively each of the properties such as its pressure (`p`), volume (`V`), and temperature (`T`) as well as the composition of the system.
● We need to describe the system by specifying it before and after the change.
`=>` You would recall from your Physics course that the state of a system in mechanics is completely specified at a given instant of time, by the position and velocity of each mass point of the system.
`=>` In thermodynamics, a different and much simpler concept of the state of a system is introduced.
● It does not need detailed knowledge of motion of each particle because, we deal with average measurable properties of the system.
● We specify the state of the system by `color{red}("state functions")` or `color{red}("state variables")`.
● The state of a thermodynamic system is described by its measurable or macroscopic (bulk) properties.
`=>` We can describe the state of a gas by quoting its pressure (`p`), volume (`V`), temperature (`T`), amount (`n`) etc.
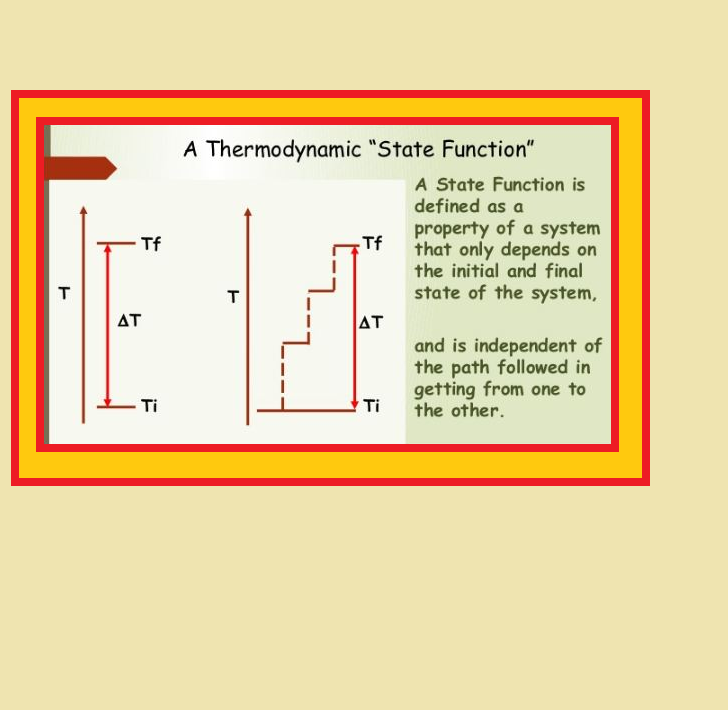
● Variables like `p`, `V`, `T` are called `color{red}("state variables")` or `color{red}("state functions")` because their values depend only on the state of the system and not on how it is reached.
`=>` In order to completely define the state of a system it is not necessary to define all the properties of the system; as only a certain number of properties can be varied independently.
● This number depends on the nature of the system. Once these minimum number of macroscopic properties are fixed, others automatically have definite values.
`color{red}("Note ")` The state of the surroundings can never be completely specified; fortunately it is not necessary to do so.
● We need to describe the system by specifying it before and after the change.
`=>` You would recall from your Physics course that the state of a system in mechanics is completely specified at a given instant of time, by the position and velocity of each mass point of the system.
`=>` In thermodynamics, a different and much simpler concept of the state of a system is introduced.
● It does not need detailed knowledge of motion of each particle because, we deal with average measurable properties of the system.
● We specify the state of the system by `color{red}("state functions")` or `color{red}("state variables")`.
● The state of a thermodynamic system is described by its measurable or macroscopic (bulk) properties.
`=>` We can describe the state of a gas by quoting its pressure (`p`), volume (`V`), temperature (`T`), amount (`n`) etc.
● Variables like `p`, `V`, `T` are called `color{red}("state variables")` or `color{red}("state functions")` because their values depend only on the state of the system and not on how it is reached.
`=>` In order to completely define the state of a system it is not necessary to define all the properties of the system; as only a certain number of properties can be varied independently.
● This number depends on the nature of the system. Once these minimum number of macroscopic properties are fixed, others automatically have definite values.
`color{red}("Note ")` The state of the surroundings can never be completely specified; fortunately it is not necessary to do so.